Development progress
LT3001 has successfully completed 2 Phase 2b clinical trials across the U.S., EU, China, and Taiwan, demonstrating strong efficacy and a favorable safety profile with no hemorrhagic complications, while offering an extended therapeutic window of up to 24 hours post-stroke onset. Lumosa is preparing a two-prong development strategy for LT3001 - in the US and China, Phase 3 trials will be conducted in the AIS patients left with no treatment options to expand the potential treatable population. The development and commercial rights in China have been granted to Shanghai Pharmaceuticals.
Clinical Trials
- Stage
- Description
- Region
- Status
- Link
-
Stage :
Phase 1 (LT3001-101)
-
Description :
Single dose on healthy volunteers
-
Region :
USA
-
Status :
Completed
-
Link :
TBA
-
Stage :
Phase 1 (LT3001-103)
-
Description :
Multiple dose on healthy volunteers
-
Region :
China
-
Status :
Completed
-
Link :
-
Stage :
Phase 1 (LT3001-105)
-
Description :
Multiple dose on healthy volunteers
-
Region :
USA
-
Status :
Completed
-
Link :
-
Stage :
Phase 2a (LT3001-201)
-
Description :
Single dose on AIS patients
(within 24 hrs)
-
Region :
USA/Taiwan
-
Status :
Completed
-
Link :
-
Stage :
Phase 2 (LT3001-202)
-
Description :
Multiple dose on AIS patient
(within 24 hrs)
-
Region :
China
-
Status :
Completed
-
Link :
-
Stage :
Phase 2 (LT3001-203)
-
Description :
Multiple dose with thrombectomy on AIS patient
(within 24 hrs)
-
Region :
USA/Taiwan
-
Status :
Ongoing
-
Link :
-
Stage :
Phase 2 (LT3001-205)
-
Description :
Multiple dose on AIS patient
(within 24 hrs)
-
Region :
USA/Taiwan/EU
-
Status :
Completed
-
Link :
LT3001-Related Studies
Jiang, Y., Ji, Y., Zhou, I. Y., et al. (2024). Effects of the new thrombolytic compound LT3001 on acute brain tissue damage after focal embolic stroke in rats. Translational Stroke Research, 15, 30–40. https://doi.org/10.1007/s12975-022-01107-3
Chao, A. C., Lee, T. H., Pettigrew, L. C., Hannawi, Y., Huang, H. Y., Chi, N. F., Chan, L., Chen, P. L., & Devlin, T. (2024). Intravenous odatroltide for acute ischemic stroke within 24 hours of onset: A phase 2, multicenter, randomized, double-blind, placebo-controlled study. Drug Design, Development and Therapy, 18, 2033–2042. https://doi.org/10.2147/DDDT.S460831
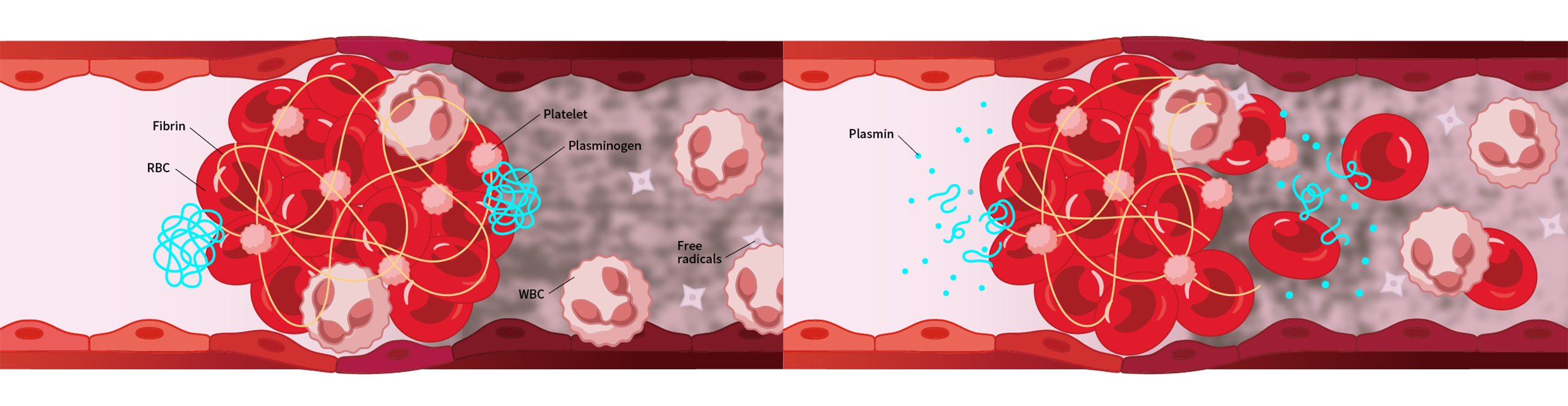
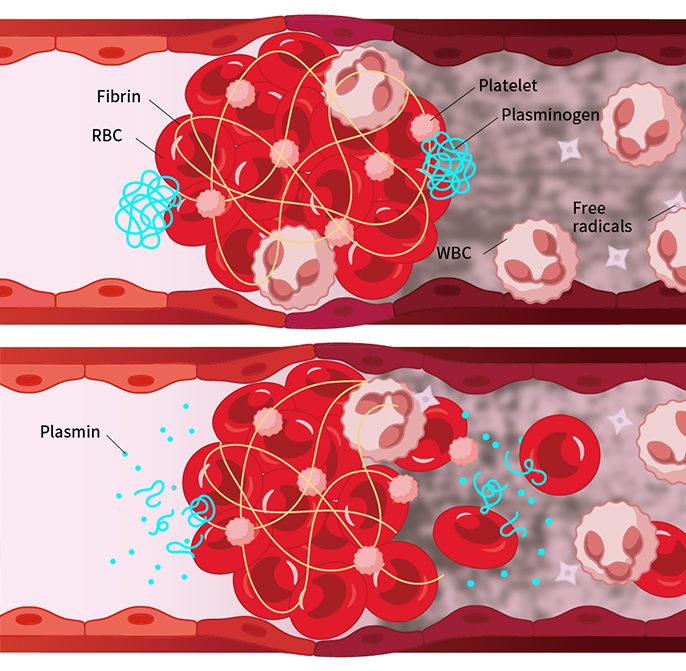 1
1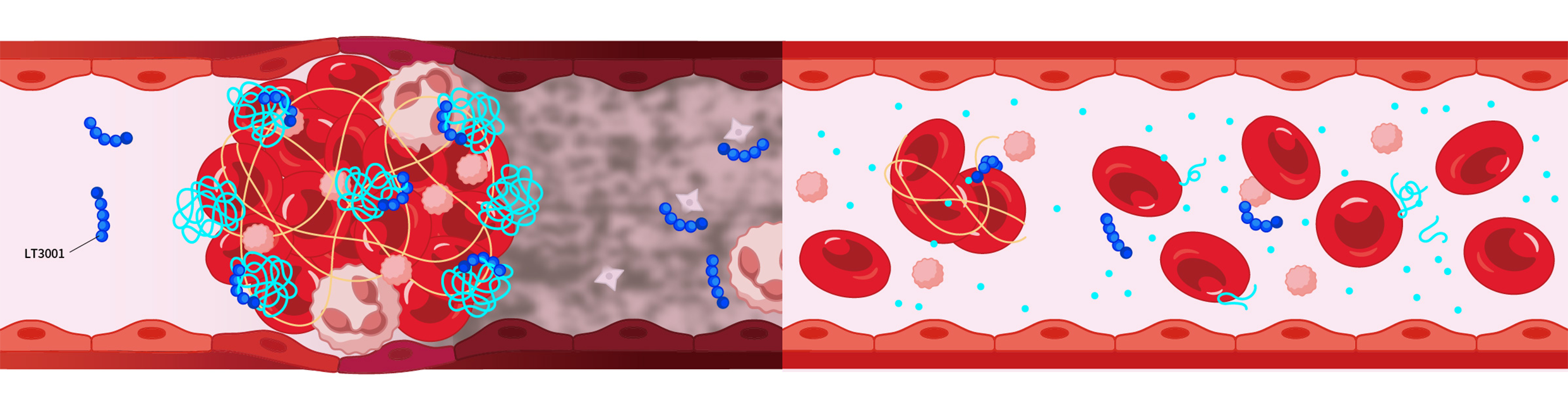
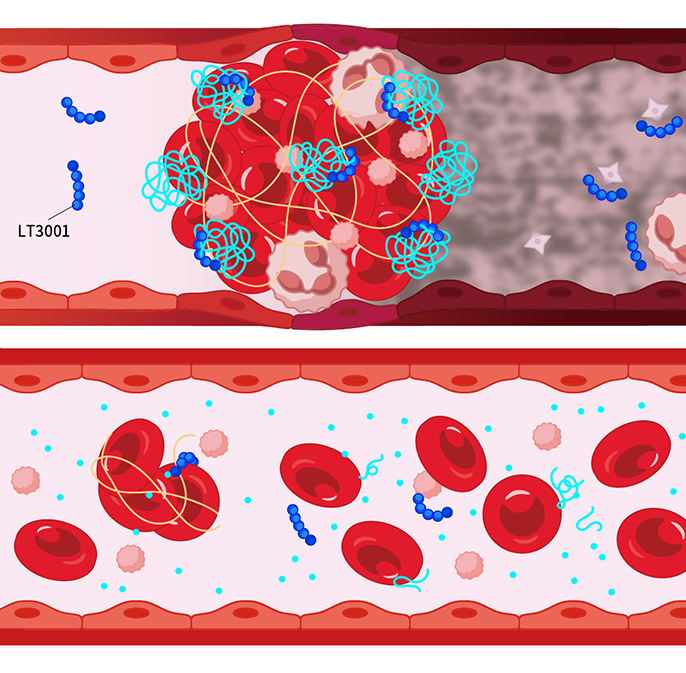 2
2





